+86 13600513715
+86 13600513715 Water Treatment Technologies in RAS
Introduction
Recirculating Aquaculture Systems (RAS) employ a suite of integrated Water Treatment technologies to maintain the precise water quality parameters required for intensive aquaculture operations. The system's effectiveness hinges on addressing five critical treatment areas: the removal of suspended solids through mechanical and biological filtration processes, the elimination of toxic ammonia nitrogen via biofiltration and nitrification, the stripping of accumulated carbon dioxide through specialized gas exchange units, comprehensive disinfection using ultraviolet irradiation or ozonation to control pathogens, and advanced oxygenation techniques to sustain optimal dissolved oxygen levels.
These interconnected technologies work synergistically to create a stable aquatic environment that supports high stocking densities while enabling water reuse rates exceeding 90%, significantly reducing both water consumption and environmental discharge compared to conventional aquaculture systems. The proper integration of these treatment components is essential for maintaining fish health, optimizing growth performance, and ensuring the overall economic viability of RAS operations.

Five Key Technologies
1. Suspended Solids and Treatment Technologies
Suspended solids in industrialized aquaculture systems primarily originate from feed inputs. Depending on feeding rates, their concentration typically ranges from 5 to 50 mg/L. With a feed conversion ratio (FCR) of 0.9–1.0, 150–200 grams of suspended solids are generated for every 1 kilogram of fish weight gain. Consequently, suspended solids accumulate rapidly in recirculating Aquaculture Water.
Under normal metabolic conditions, fish excrete solid waste in the form of suspended particles. In flowing aquaculture water, most suspended solids exist as particles smaller than 30 microns (μm). These particles have a density slightly higher than water, exhibit small size, high mobility, and slight adhesiveness, remaining suspended under water flow conditions. Removing sub-30-micron suspended solids has long been a critical focus in the design of industrialized aquaculture systems.
The accumulation of suspended solids increases water turbidity, impairs respiratory efficiency in cultured species, elevates environmental stress, degrades water quality, and depletes dissolved oxygen(DO). Thus, timely removal ofsuspended solids is essential for maintaining optimal water conditions in industrialized aquaculture operations.
①Fixed-Bed Filtration
Fixed-bed filters typically consist of coarse, medium, and fine filter media layers. Based on water flow dynamics, they are categorized into:
- Spray-type filters
- Pressure-type filters
Advantages: Adjustable media granularity and bed thickness allow targeted removal of suspended particles.
Challenges: Large footprint, low efficiency, prone to clogging during prolonged operation, and difficult backwashing.
②Screen Filtration
Screen filtration employs fine mesh (30–100 μm / 0.03–0.1 mm) to remove suspended solids, with two main types:
- Static (flat-panel) screens
- Rotary Drum Screens (more efficient, with continuous backwashing)
Performance:
- Removes 36–67% of suspended solids; smaller mesh sizes (<60 μm) improve removal but reduce flow rates.
Research Focus: Optimizing mesh geometry to increase surface area, prevent clogging, and minimize backwash water usage.
③Floating-Bed Filtration
Uses low-density plastic spheres (e.g., 3 mm diameter) as buoyant filter media.
Efficiency:
- 100%removal of particles >30 μm
- 79%removal of particles <30 μm
Challenges: Frequent backwashing required due to particle agglomeration, increasing water and operational costs.
Future Work: Anti-clogging designs and improved backwash methods.
④Natural Sedimentation
Leverages settling ponds or tank designs to aggregate and remove suspended solids.
Key Parameters:
- Flow velocity: Optimal ≤1 m/min (max 4 m/min)
- Hydraulic loading rate: 1.0–2.7 m³/(m²·h)
Efficiency: Removes 59–90% of suspended solids, but large infrastructure is needed to accommodate low flow rates.
⑤Dissolved Air Flotation (DAF)
Principle: Microbubbles (10–100 μm) act as a "screening" layer, adsorbing suspended solids via surface tension.
Critical Factor: Smaller bubbles (e.g., nanobubbles) significantly improve efficiency.
Application: Effective for high-load suspended solids, but relies on advanced bubble generation technology.
2. Ammonia Nitrogen and Its Treatment Technologies
Ammonia nitrogen in recirculating aquaculture systems primarily originates from fish metabolism, residual feed, and decomposition of organic matter. Only 7-32% of the total nitrogen excreted by cultured fish is contained in suspended solids, while the majority dissolves in the water column, existing in both ionized ammonium (NH₄⁺ ) and unionized ammonia (NH₃) forms that interconvert depending on pH and temperature variations. Effective ammonia nitrogen management in recirculating water through physical, chemical, and biological treatment methods represents a critical factor for advancing intensive aquaculture production.
The accumulation of ammonia nitrogen exerts toxic effects on fish, with unionized ammonia (NH₃) being particularly harmful. In intensive aquaculture systems, the total ammonia nitrogen concentration should generally not exceed 1 mg/L, while the unionized ammonia concentration should remain below 0.05 mg/L. Given the pH- and temperature-dependent equilibrium between NH₄⁺ and NH₃, proper pH adjustment according to temperature fluctuations is essential to maintain unionized ammonia at minimal concentrations while controlling overall ammonia accumulation.

① Air Stripping Treatment
Principle:
Air stripping operates based on gas-liquid phase equilibrium and Henry's Law of mass transfer. Under intense aeration, the partial pressure of dissolved gases is reduced, allowing ammonia in the water to volatilize across the gas-liquid interface and transfer into the air, thereby achieving ammonia nitrogen removal.
Key Factors:
- pH dependence:
At higher pH, most ammonia nitrogen exists as unionized ammonia (NH₃), which is more volatile.
- Optimal conditions:
At pH 11.5 with an air-to-water volume ratio of 1:107, 95% of ammonia nitrogen can be removed.
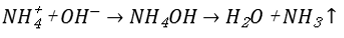
Operational Considerations:
- pHadjustment:
Critical for balancing treatment efficiency and fish safety (avoiding extreme pH stress).
- High air flow requirement:
Large volumes of air are needed, which may affect water temperature stability in aquaculture systems.
②Ion Exchange Adsorption
Ion exchange adsorption utilizes zeolite or exchange resins to remove ammonia nitrogen from water. Zeolite exhibits an adsorption capacity of approximately 1 mg/g. With proper design, it can remove up to 95%of ammonia nitrogen. When saturated, the medium can be regenerated through 24-hour spraying with 10% saline solution for reuse. While zeolite demonstrates good performance in intensive aquaculture systems, its regeneration process is cumbersome and time-consuming.
③Biological Bacterial Treatment
Biological treatment employs nitrifying bacteria (Nitrosomonas), nitrite-oxidizing bacteria (Nitrobacter), and denitrifying bacteria to convert and remove ammonia nitrogen. The process involves:
- Ammonia oxidation to nitrite by Nitrosomonas
- Nitrite oxidation to nitrate by Nitrobacter
- Optional denitrification to nitrogen gas under anaerobic conditions (DO <1 mg/L)
Key characteristics:
- Nitrate exhibits low toxicity to fish (some species tolerate >200 mg/L)
- Typical conversion rate: 380 g/(m³·d) of ammonia nitrogen
- Feed load capacity: 32 kg/(m³·d)
- Optimal temperature >30°C (activity declines below 15°C)
④Ozone Oxidation Treatment
While primarily used for disinfection and suspended solids removal, ozone demonstrates moderate ammonia oxidation capacity:
- Direct oxidation removes 25.8% of ammonia nitrogen
- Catalytic oxidation significantly improves efficiency
This promising approach requires further research for aquaculture applications.
⑤Aquatic Plant Treatment
Tail water passes through hydroponic vegetable channels where plant root systems create unique microbial ecosystems that cost-effectively remove ammonia nitrogen, nitrites, and other harmful compounds.
3. CO₂ Stripping Treatment
The primary harmful gas in recirculating aquaculture systems is carbon dioxide (CO₂) produced by fish respiration and metabolism, which exists as microbubbles in water. Elevated CO₂ concentrations (>20 mg/L) cause gas pressure stress in fish, manifesting as:
- Surface crowding near aeration devices
- Significant reduction in feeding activity
CO₂ reacts reversibly with water to form carbonic acid (H₂CO₃), a weakacid that lowers system pH and deteriorates water quality. Being highly unstable, carbonic acid readily decomposes into water and CO₂ when exposed to air. Effective CO₂ removal therefore requires maximizing water-air contact.
①Mechanical Removal (Aeration)
- Utilizes aerators or diffusers to create vertical water circulation
- Enhances atmospheric exchange for carbonic acid decomposition
- Standard method in most RAS operations
②Hydraulic Design Removal
- Incorporates elevation drops between return pipes and channels
- Creates waterfall effects to maximize air exposure during water return
- Passive approach requiring careful hydraulic engineering
③Microbubble Stripping
- Installs fine bubble diffusers along water channels
- Leverages bubble coalescence to capture dissolved CO₂
- Rising bubbles transport CO₂ to surface for atmospheric release
- Particularly effective for high-density systems
Key Parameters:
- Critical threshold: 20 mg/L CO₂ (behavioral stress observable)
- Optimal range: <10-15 mg/L for most cultured species
- pH interaction: Every 10 mg/L CO₂ ≈ 0.1 pH unit decrease
4. Disinfection and Sterilization
In intensive aquaculture systems, high stocking densities and substantial feed inputs lead to the accumulation of nutrients in the water, creating ideal conditions for bacterial growth. Without timely disinfection, disease outbreaks can occur and spread rapidly under high-density conditions, potentially causing catastrophic losses. Therefore, integrating effective sterilization equipment into system design is essential. The primary disinfection methods include ozonation and ultraviolet (UV) irradiation.
①Ozone Disinfection
Ozone (O₃) is a highly unstable and powerful oxidizing agent that destroys the cell membranes of bacteria, viruses, and parasites at specific concentrations. Studies indicate that maintaining ozone levels at 0.1–0.2 mg/L for 1–30 minutes achieves optimal sterilization.
Additional Benefits:
- Precipitates suspended solids
- Oxidizes ammonia nitrogen (NH₃/NH₄⁺ )
If its multifunctional efficiency is improved, ozone could see broader application in intensive aquaculture.
②Ultraviolet (UV) Disinfection
Research confirms that UV light at specific wavelengths (180–300 nm) effectively sterilizes pathogens.
Key Parameters:
- Required UV intensity: 15,000–30,000 µW/cm²
- At 30,000 µW/cm², UV exposure achieves:
- 100%inactivation of shrimp white spot virus (WSSV) in 2.67 seconds
- Elimination of saprolegniasis (water mold) and viral hemorrhagic septicemia (VHS) in 1.60 seconds
Hybrid UV-Ozone Systems:
Some studies have tested UV-ozone generators, which produce ozone alongside UV disinfection, enabling simultaneous sterilization and ammonia oxidation for enhanced efficiency.
5. Oxygenation Technologies
Dissolved oxygen (DO) in aquaculture water is essential for fish survival and microbial activity in treatment systems. In intensive aquaculture systems, DO levels should be maintained at:
- ≥60%ofsaturation or > 5 mg/L for normal fish growth
- <2mg/L: Nitrifying bacteria lose their ammonia-oxidizing function
Primary Oxygen Consumers:
- Fish respiration
- Decomposition of metabolic waste
- Microbial ammonia treatment
Oxygen demand varies with species, stocking density, and feeding rates, requiring tailored oxygenation strategies in system design.
①Aeration (Air-Based Oxygenation)
Due to limited space for mechanical aerators, blower + diffuser systems are commonly used, generating small bubbles for oxygenation.
Characteristics:
- Advantages: Low-cost, easy operation
- Limitations: Lowefficiency (~1.300 kg O₂/kWh at20°C;drops to 0.455 kg O₂/kWh at 28°C)
- Maxstocking density: 30–40 kg/m³
Common Aeration Methods:
- Diffused aeration (e.g., fine-bubble diffusers)
- Paddlewheel aerators
- Surface agitators (e.g., propeller aspirators)
②Pure Oxygen Injection
Oxygen Sources:
- Compressed oxygen cylinders
- Liquid oxygen tanks
- On-site oxygen generators
Challenges of Traditional Methods:
- Low efficiency (≤40% utilization) due to oxygen escape
- Requires specialized equipment for optimal dissolution
Pressure Saturation Method (High-Efficiency Solution):
- High-pressure mixing: Water and O₂ saturate under pressure.
- Release to atmospheric pressure: Creates supersaturated DO water (~90% O₂ utilization).
- Molecular diffusion: Oxygen permeates surrounding water.
Max stocking density: Up to 100 kg/m³
③Microbubble Oxygenation
Technology:
- Ultrasonic bubble fragmentation produces <20 μm microbubbles, enhancing gas transfer efficiency.
Advantages:
- Higher DO absorption rates
- Improved oxygen utilization
Limitations:
- High operational costs
- Technical complexity
Conclusion
In summary, RAS water treatment integrates multiple technologies to address suspended solids, toxic nitrogen compounds, CO₂ accumulation, microbial risks, and oxygen demand. By optimizing these systems, RAS achieves efficient water reuse, reduces environmental impact, and supports high-density aquaculture. Future advancements will further enhance their precision and energy efficiency.