+86 13600513715
+86 13600513715 Effects of Mn²⁺Concentration Gradients on MBBR Performance
Effects of Mn²⁺Concentration Gradients on MBBR Performance
Application of Manganese in Nitrogen and Phosphorus WasteWater Treatment
Manganese (Mn), an essential trace element, plays a vital role in biogeochemical processes and is the second most abundant transition metal in the Earth's crust (0.1%) after iron. In Moving Bed Biofilm Reactors (MBBRs), Mn-mediated redox cycling has proven effective in removing micropollutants, including antibiotics and antidepressants. The coupling of Mn-driven mixotrophic denitrification with organic degradation has attracted significant attention for nitrogen removal.
The redox transition between Mn²⁺ and Mn⁴⁺ promotes the enrichment of denitrifying bacteria and functional genes, enhancing pollutant removal. Integrating MBBR with Mn cycling achieves 84.7% total nitrogen (TN) removal efficiency, while significantly altering microbial communities to facilitate simultaneous nitrogen and organic matter degradation. Studies show that manganese oxides (MnOx) and Mn²⁺ participate in nitrogen transformation, where MnOx can oxidize NH₄⁺-N to NO₃⁻-N.
Mechanisms of Mn-Mediated Nitrogen Removal
- Nitrification & Manganese Reduction
- MnOx reduction coupled with nitrification, mediated by Mn-reducing bacteria, yields NO₃⁻-N and NO₂⁻-Nas end products.
- Unlike conventional nitrification, anaerobic ammonium oxidation(anammox) directly converts NH₄⁺-N to N₂ under anoxic conditions using MnOx as an electron acceptor.
- Denitrification & Mn Redox Coupling
- NO₃⁻-N and NO₂⁻-N from nitrification can couple with re-oxidation of reduced MnOx, enhancing TN removal.
- Mn²⁺ and its oxidation byproductsserve as electron donors for denitrification, simultaneously removing Mn²⁺ from wastewater.
- Microbial Mn²⁺ Oxidation
- Microbial oxidation of Mn²⁺ to biogenic MnOx (BioMnOx) is driven by heterotrophic and autotrophic Mn-oxidizing bacteria, with heterotrophs (e.g., Cupriavidus sp. HY129) playing a dominant role.
- Enzymes such as multicopper oxidases (MCOs)and animal heme peroxidases (AHPs) catalyze Mn²⁺ oxidation.
- BioMnOx exhibits superior oxidative capacity compared to natural MnOx, effectively degrading antibiotics (e.g., tetracycline, ciprofloxacin, sulfamethoxazole).
Mn-Driven Phosphorus Removal
Unlike nitrogen, phosphorus cannot be converted into gaseous forms and is primarily removed via immobilization.
- MnOx Adsorption: MnOx acts as a strong adsorbent for phosphate (PO₄³⁻).
- Mn²⁺-Phosphate Precipitation: During MnOx reduction, released Mn²⁺ reacts with phosphate to form insoluble Mn₃(PO₄)₂.
- BioMnOx Regeneration: In anaerobic-aerobic MBBRs, BioMnOx regenerated from Mn²⁺ oxidation exhibits enhanced adsorption capacity for micropollutants.
Key Findings & Applications
- Optimal Mn²⁺ Concentration: Cupriavidus sp. HY129 achieves 8% NO₃⁻-N and 87.2% Mn²⁺ removal, but excessive Mn²⁺ may cause NO₂⁻-N accumulation.
- Robust Mn²⁺ Removal in MBBR: BioMnOx generated in MBBR continuously adsorbs Mn²⁺, unaffected by initial Mn²⁺ shocks.
- Enhanced Pollutant Removal: Adding Mn²⁺ to MBBR improves simultaneous NH₄⁺-N, NO₃⁻-N, COD, and TP removal, making it a promising strategy for wastewater treatment.
Effects of Mn²⁺ Concentration on Simultaneous Nitrogen and Manganese Removal in MBBR Systems
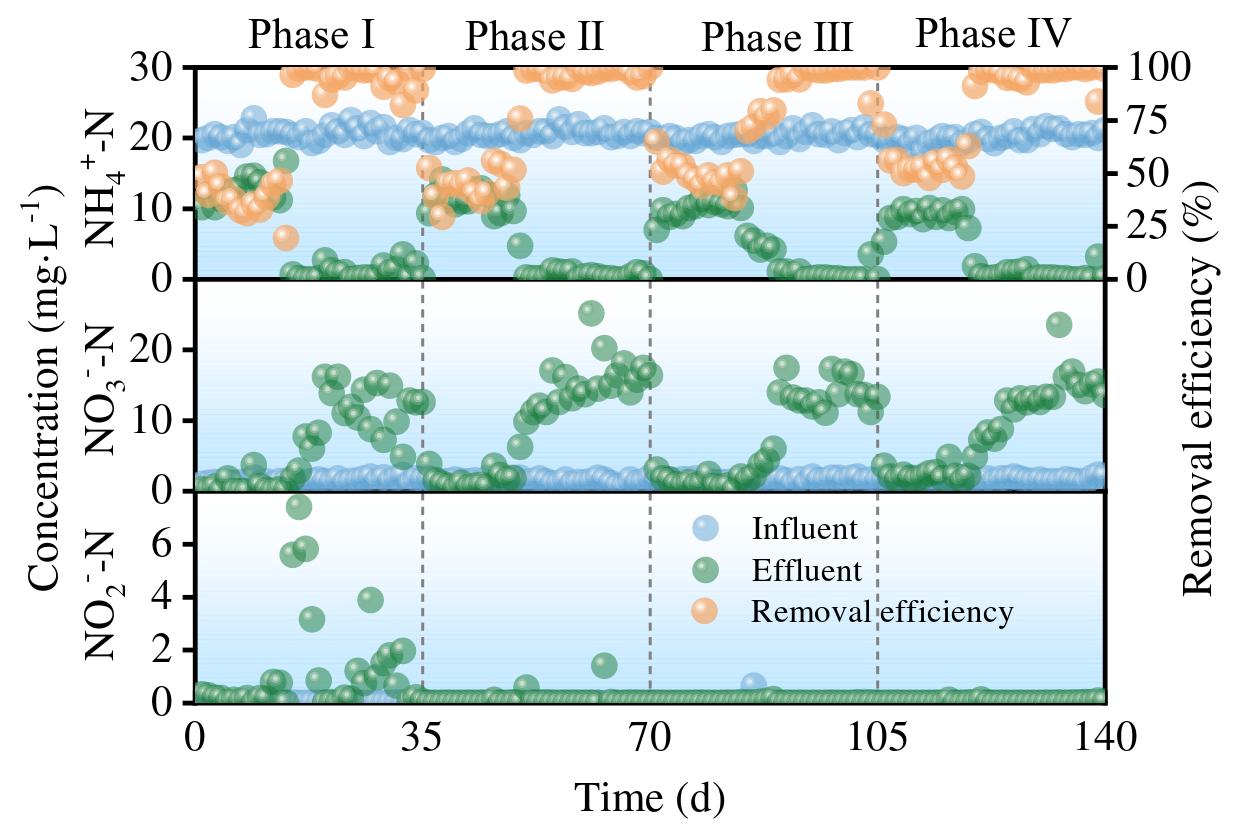
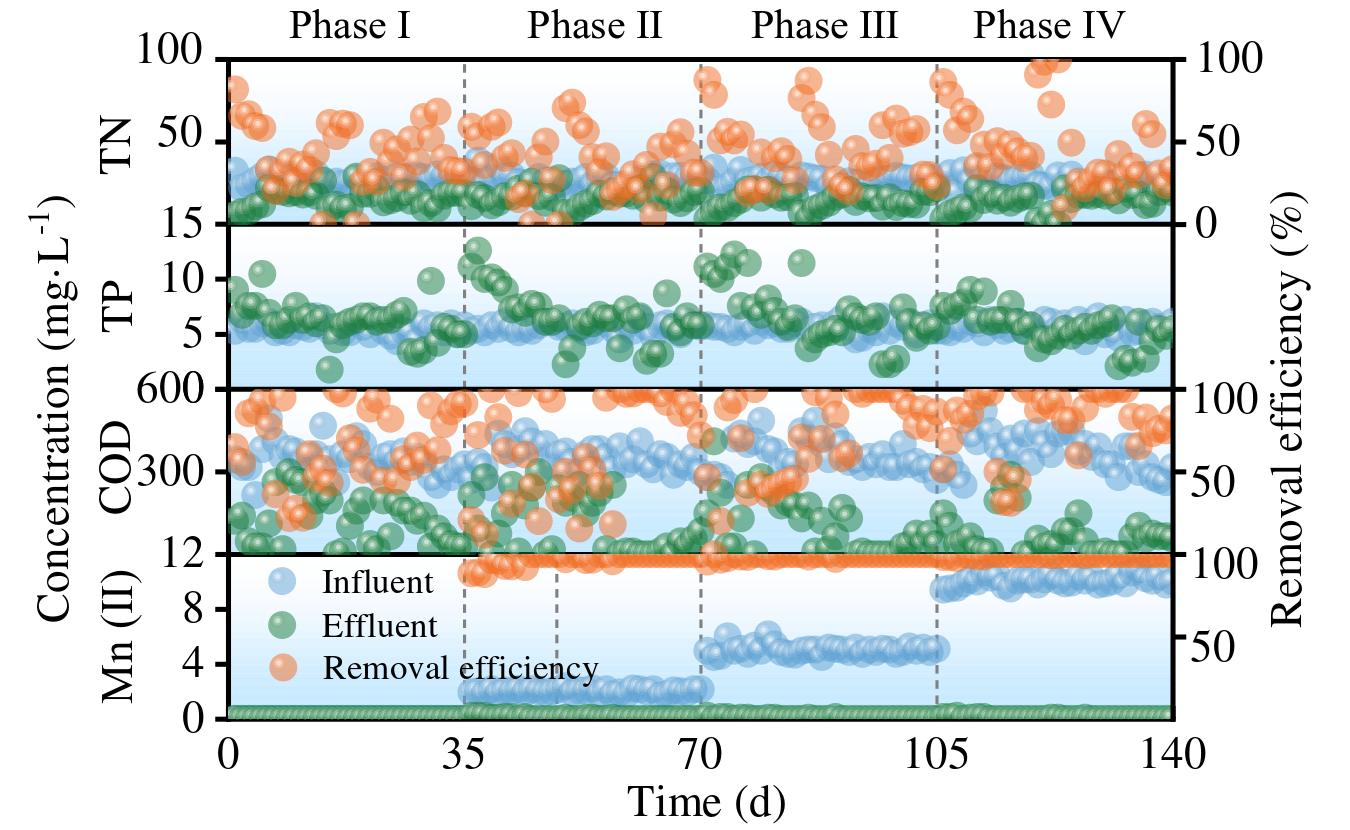
The MBBR system coupled with manganese redox cycling demonstrates effective nutrient removal (nitrogen and phosphorus) through biologically mediated redox reactions. As shown in Figures below, the system maintained stable performance in treating NH₄⁺-N, NO₃⁻-N, NO₂⁻-N, TN, TP, COD, and Mn²⁺ across four operational phases.
Key Findings
- NH₄⁺-N Removal Efficiency
- At Mn²⁺ concentrations of 0, 2, 5, and 10 mg/L, average NH₄⁺-N removal efficiencies were 85%, 75.92%, 75.27%, and 80.21%, respectively.
- Optimal performancewas observed at 10 mg/L Mn²⁺, with an 21% removal rate (7.36% increase vs. control).
- Results confirm that Mn²⁺ enhances nitrification efficiency in MBBR biofilms.
- TN Removal Efficiency
- TN removal rates: 53% (0 mg/L), 37.58% (2 mg/L), 45.87% (5 mg/L), and 47.42% (10 mg/L).
- Peak efficiency (47.42%)occurred at 10 mg/L Mn²⁺, indicating improved denitrification.
- COD Removal Efficiency
- Without Mn²⁺: 77% removal(344.87 → 119.13 mg/L).
- With Mn²⁺: 96% (2 mg/L), 70.51% (5 mg/L), and 80.95% (10 mg/L).
- Mn²⁺ addition significantly boosts COD degradation.
- Mn²⁺ Removal Efficiency
- Near-complete elimination: 97.76% (2 mg/L), 99.42% (5 mg/L), 99.68% (10 mg/L).
- BioMnOx formation enables continuous Mn²⁺ adsorption, even under shock loads.
- TP Removal Efficiency
- Lowest effluent TP (5.62 mg/L)at 10 mg/L Mn²⁺, suggesting enhanced PAO activity.
- NO₃⁻-N & NO₂⁻-N Dynamics
- NO₃⁻-N accumulationincreased with Mn²⁺ dosing (likely due to nitrification stimulation).
- NO₂⁻-N levelsremained low (0.02–1.13 mg/L), indicating stable partial denitrification.
Conclusions
- Optimal Mn²⁺ dosage: 10 mg/L, maximizing TN (47.42%), NH₄⁺-N (80.21%), and COD (80.95%) removalwhile minimizing TP.
- Mechanisms:
- Mn²⁺ → BioMnOx conversion enhances nitrogen removaland heavy metal adsorption.
- Mn redox cycling promotes synergistic NH₄⁺-N and COD degradation.
- Robustness: MBBR withstands Mn²⁺ fluctuations without performance inhibition.